WELCOME TO ADCURE BIO
AdCure Bio is a pre-clinical stage cancer viro-immuno-therapy company, developing potent and safe immuno-stimulatory oncolytic viruses for therapy of solid tumors.
AdCure Bio is committed to bring patients new therapies for late-stage and disseminated metastatic cancers, refractory to immunotherapy, chemotherapy and other existing treatment modalities.
THE DIFFERENCE IN ADCURE BIO TECHNOLOGY
Our proprietary platform adenovirus configuration is engineered to safely reach and destroy cancer cells and stimulate anti-tumor immunity within non-resectable and metastatic tumors after intravenous virus administration.
Unmet need for novel Therapeutics to cure cancer
Cancer figures among the leading causes of morbidity and mortality worldwide, with approximately 14 million new cases and 9.6 million cancer related deaths in 2018.
The total annual economic cost of cancer in 2010 was estimated at approximately US$ 1.16 trillion. Unfortunately, the number of new cancer cases is expected to rise by about 70% over the next 2 decades*.
Oncolytic virotherapy is a new modality for therapy of hematologic and solid cancers. As of 2018, oncolytic viruses are being evaluated in 112 clinical trials as novel anti-cancer therapeutics**.
* WHO Cancer Statistics http://www.who.int/mediacentre/factsheets/fs297/en/
** ClinicalTrials.gov
AdCure clinical program
AdCure Bio's clinical development program is focused on developing new viruses that can efficiently reach disseminated cancer cells via intravascular virus delivery.
Currently, our main focus is on lung cancer, the deadliest cancer type in the US, Europe, and Asia. The American Cancer Society estimates that in the US in 2019, there will be 205,335 new cases and 128,403 deaths due to the non-small cell lung cancer, the most prevalent lung cancer type.
AdCure Bio's lead products: AVID-212 and AVID-388
The key technology platform of AdCure Bio is based on oncolytic adenovirus, a common cold virus, engineered to selectively infect and kill tumor cells and stimulate the patient's immune system to eliminate cancer cells from the body.
AdCure Bio’s one-of-a-kind Artificial Vectors for Intravascular Delivery - AVID platform - is ideal for simple intravascular administration because of its safety and unique property of escaping liver sequestration. These features make it suitable for the treatment of cancer patients with disseminated metastatic disease and non-resectable tumors, for whom no effective therapy is currently available.
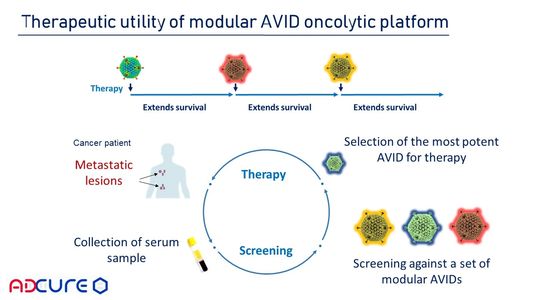
Based on the available variants of individual AVID capsid proteins, a panel of immunologically distinct oncolytic AVID variants can be assembled for repeated rounds of therapy to improve anti-tumor efficacy and extend survival again and again.
NEWS and Presentations
Press Release
AdCure Bio is pleased to announce a landmark publication describing the design, development, and evaluation of the oncolytic viruses for intravenous administration in pre-clinical models of localized and disseminated cancer. The study has been published in the journal Science Translational Medicine on November 25th, 2020. Please follow the journal link for more information: https://stm.sciencemag.org/content/12/571/eabc6659
International Oncolytic Virus Conference, October 9-12th, 2019. Rochester, MN
Structure-guided engineering of oncolytic adenovirus for systemic cancer therapy. (Plenary Session talk)
Press Release
On August 13, 2019, The United States Patent and Trademark Office granted AdCure utility United States Patent No. 10,376,549 titled "Detargeted adenovirus variants and related methods".
American Society of Gene and Cell Therapy conference, April 29, 2019. Washington DC.
Pre-clinical assessment of efficacy and safety of novel oncolytic adenovirus for therapy of disseminated lung cancer. (Poster presentation)
American Association for Cancer Research conference, April 3, 2019. Atlanta, GA
Pre-clinical assessment of efficacy and safety of novel oncolytic adenovirus for therapy of disseminated lung cancer. (Poster presentation)
Biotech Showcase-2019 / 37th JP Morgan Healthcare Conference, January 9, 2019. San Francisco, CA
Novel oncolytic AVID platform for systemic therapy of solid cancers. (Oral presentation)
Press Release
On May 29, 2018, The United States Patent and Trademark Office granted AdCure utility United States Patent No. 9982276, titled "Penton-mutated, integrin-retargeted adenovirus vectors with reduced toxicity and their use".
AdCure BIo's pipeline
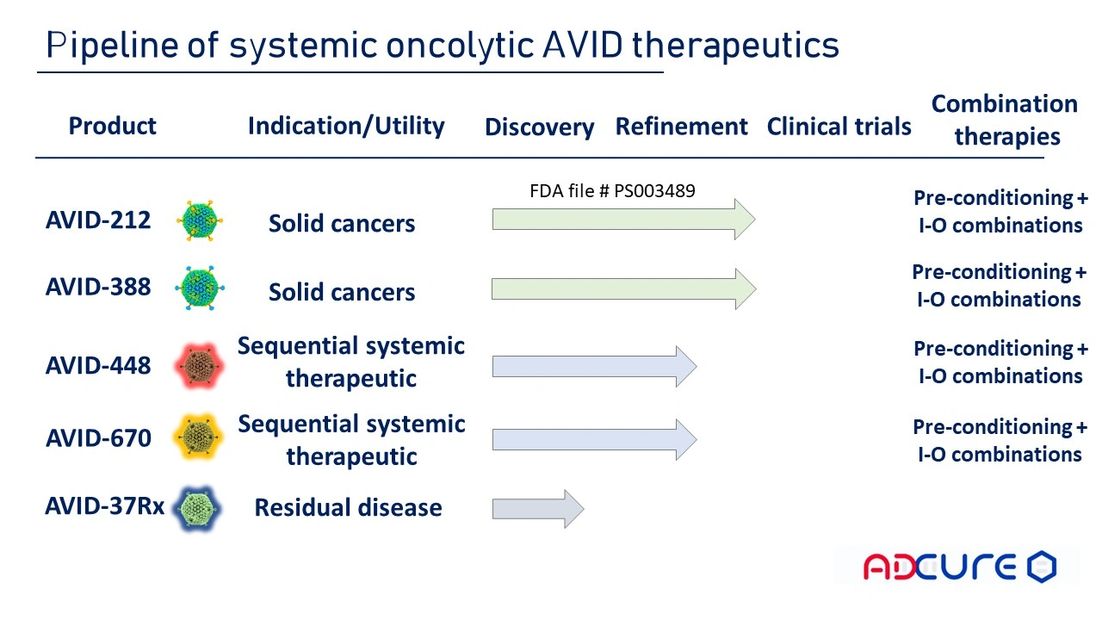
Using its versatile AVID platform allowing for a safe intravenous virus delivery, AdCure Bio continues to innovate and develop novel oncolytic adenovirus therapeutics to further increase virus potency resistance to pre-existing immunity, and stimulation of patient's own immune system to attack and eliminate cancer cells.
Intellectual property

AdCure Bio owns unrestricted rights to its core therapeutic virus technology
U.S. Patent 9,982,276
Title: "Penton-mutated, integrin-retargeted adenovirus vectors with reduced toxicity and their use".
Issue date: May 29, 2018. Priority date: March, 12, 2015
This patent covers configuration of adenoviruses with modified penton for safe therapeutic gene delivery after intravenous virus administration.
U.S. Patent 10,376,549
Title: "Detargeted Adenovirus Variants and Related Methods".
Priority date: January, 20th, 2015
This U.S patent and international patent applications cover adenovirus configurations suitable for intravenous administration and de-targeted from the liver.
U.S. Continuation Patent Application Serial No. 16/460,160
U.S. Continuation Patent Application Serial No. 16/460,160 entitled: “Detargeted Adenovirus Variants and Related Methods” was filed on November 25, 2019. This continuation patent application is based on our granted US patent 10,376,549 and it expands and defines the methods and therapeutic uses of adenovirus variants, protected under our granted parental U.S patent.
adcure team
Henry Wyche
Dmitry M. Shayakhmetov, PhD
Dmitry M. Shayakhmetov, PhD

Chief Executive Officer
Dmitry M. Shayakhmetov, PhD
Dmitry M. Shayakhmetov, PhD
Dmitry M. Shayakhmetov, PhD

Co-Founder, Senior Vice-President,
Chief Scientific Officer
Nelson C. Di Paolo, PhD
Nelson C. Di Paolo, PhD
Nelson C. Di Paolo, PhD

Co-Founder, Vice-President,
Director of Toxicology and Preclinical Development
Angel A. Rivera, PhD
Nelson C. Di Paolo, PhD
Nelson C. Di Paolo, PhD

Chief Technology Officer
AdCure bio's partners
Intellectual Property Law
Intellectual Property Law
Intellectual Property Law

Our intellectual property partner
Corporate Law
Intellectual Property Law
Intellectual Property Law

Our corporate law partner
Preclinical studies
Intellectual Property Law
Preclinical studies

Research services agreement-based proof-of-concept and mechanism of action tests of AdCure Bio's oncolytic viruses are conducted in state-of-the-art laboratories of Emory University
Contact Us
AdCure Bio
AdCure Bio LLC | Century Springs West | 6000 Lake Forrest Drive, Suite 400 | Atlanta, GA 30328
Ph: (206)-334-6511 Email: info@adcurebio.com
Copyright © 2022 AdCure Bio, LLC - All Rights Reserved.
Powered by GoDaddy